Executive Summary
Urine, which contains valuable nutrients like nitrogen and phosphorus, can be applied to soil and used as fertiliser for crops. Though valuable, urine is sometimes difficult to transport and store and it does not have a pleasant odour. To overcome these issues, struvite can be produced. Struvite (MgNH4PO4•6H2O) has many of the fertilising properties of urine with several advantages: the volume and weight are reduced, it can be stored in a compact form and it is easy to handle, transport and apply- especially in a granulated form.
| In | Out |
|---|---|
Urine, Yellowwater |
Fertiliser |
What is Struvite?
Through a basic precipitation reaction, the majority of phosphorus in urine can be crystallised into a white, odourless powder: struvite (MgNH4PO4•6H2O), sometimes also called Magnesium Ammonium Phosphate Hexahydrate (M-A-P). Struvite is a bioavailable, slow-release fertiliser; it is compact and can be stored, transported and applied easily, and does not smell (see the picture below). Through struvite recovery, over 90% of phosphate can easily be removed from urine.

How is Struvite formed?
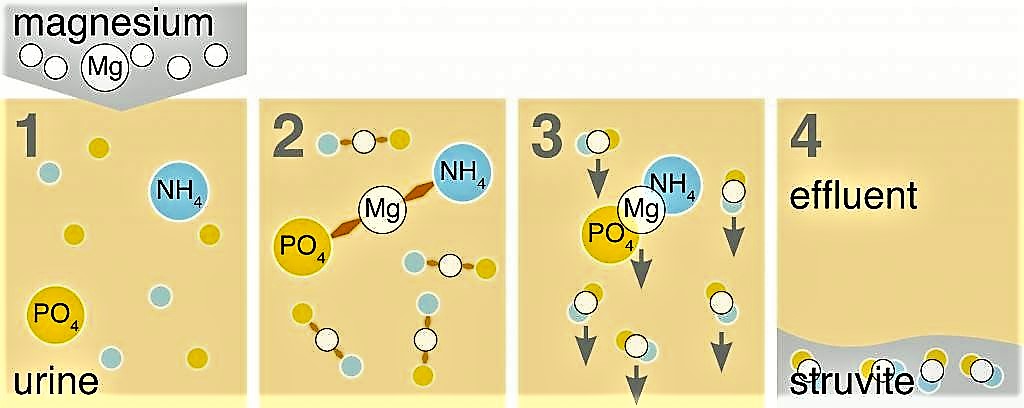
Urine contains phosphate (PO4) and ammonium (NH4). When magnesium (Mg) is added to urine, phosphate, ammonium and magnesium bind and form struvite (MgNH4PO4•6H2O), which can then be filtered out, collected, and dried into a powder (see the figure below).
Where can Magnesium for the Precipitation be found?
Various magnesium-containing substances can induce the precipitation of struvite- each with different qualities and costs. Bittern, the liquid, which remains after salt (NaCl) extraction from seawater, is highly concentrated with magnesium (and other minerals). Therefore, in coastal areas where salt is produced, this is an ideal source of magnesium. Furthermore, because it is a liquid, the bittern is easily dosed and readily mixed with urine.
Magnesite rock (MgCO3) can also be used as precipitant if it is treated properly. The rock powder is heated in a calcination kiln (analogous to lime calcination) to produce magnesium oxide (MgO), which dissolves in urine (untreated MgCO3 will not dissolve). If no local magnesium source is available, other magnesium compounds (e.g. magnesium chloride, MgCl2.6H2O or magnesium sulphate, MgSO4•7H2O), may be purchased, though they are likely to be more expensive.
How is the Struvite Reactor Built?
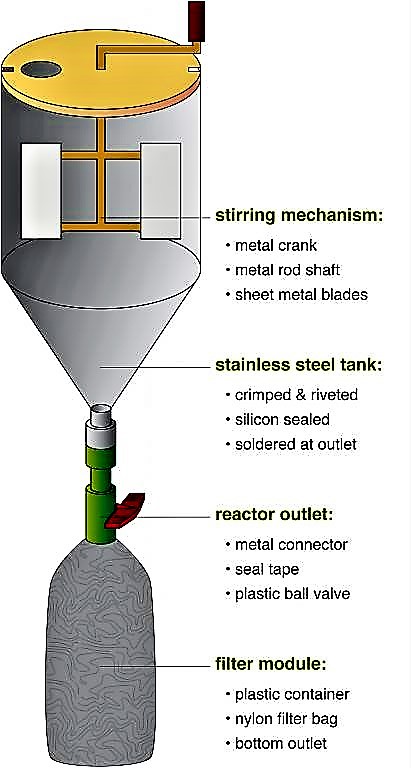
A struvite reactor can be easily built, using locally available materials and skills (see the figure below). The main tank can be constructed with conventional plastic containers (e.g. polyethylene, polypropylene, polyvinylchloride) or galvanised steel sheets. The stirring mechanism, as well as the supporting structure should be assembled from steel, which is coated or galvanised for rust proofing. Pipes and fittings should be made of plastic to prevent corrosion.
How does the Struvite Reactor work?
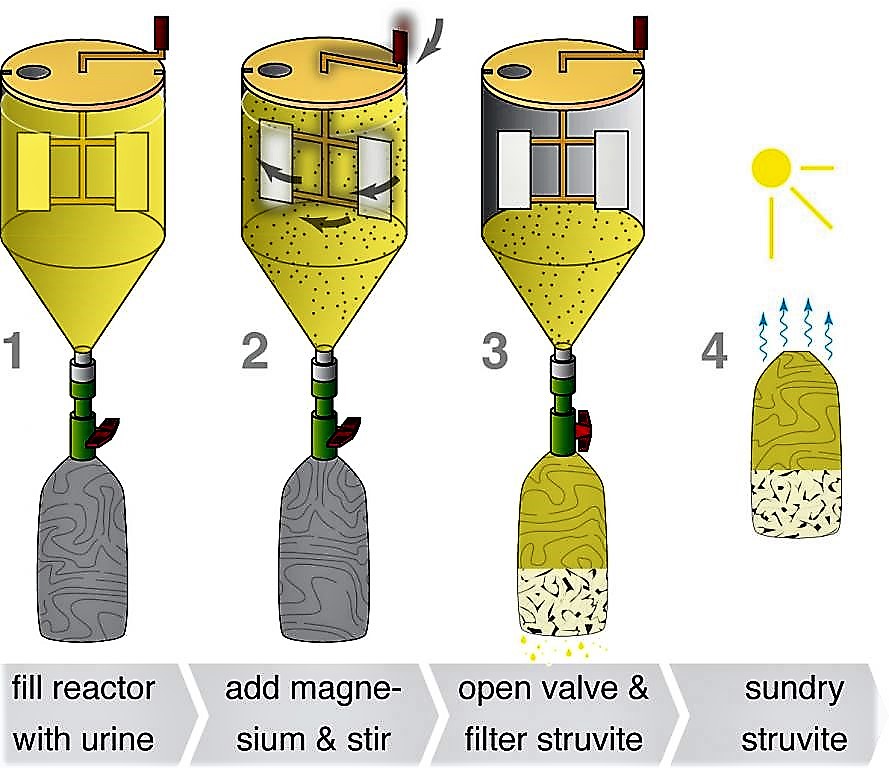
The reactor consists of a stirring mechanism, which is fitted inside the tank; a nylon cloth filter bag hangs below a valve to allow the main reactor to be drained (see the figure below). To start the process, the collected urine and magnesium are mixed for 10 minutes in the reaction tank. The valve is opened and the suspension is then drained into the filter bag. The filter bag retains the struvite while the effluent passes through. The filter bag is air dried for one to two days, after which point the struvite is ready to use. In field experiments, this type of reactor was able to recover over 90 % of the phosphate contained in the urine. Because struvite also precipitates naturally from urine, any precipitate in the collection system should be incorporated into the final product, in order to maximise nutrient recovery.
How can Struvite be Applied?
Struvite can be applied to fields just like any other fertiliser. In a simple granulation drum (see the figure below), the powder can be transformed into granules. In granular form, a fertiliser is easier to apply and does not cake in moist environments. Struvite performs comparably to diammonium phosphate (DAP) fertiliser and has the following advantages:
- Bio-available: The nutrients in struvite can be readily absorbed by the plant.
- Slow-release: Due to its low solubility, struvite guarantees a slow but steady nutrient supply.
- Highly pure: contaminants (e.g. pharmaceuticals or heavy metals), which may be present in the urine, do not precipitate with the struvite.

How can the Effluent be used?
After the struvite is filtered out of solution, the same quantity of urine remains; this effluent still contains high concentrations of nutrients, particularly nitrogen and potassium. The effluent may be used in drip-irrigation for fertigation (fertigation is the combination of fertilisation and irrigation). Further nutrient recovery technologies are being developed to extract the remaining nutrients and complement struvite precipitation.
Struvite precipitation may be appropriate for any situation where significant quantities of urine can be collected. Struvite can be produced from a variety of wastewaters (including domestic wastewater or liquid animal manure) but the process is more difficult and requires additional chemicals for pH control. Urine collection (labour) and transport (fuel) accounts for a large proportion of the costs, and therefore struvite production is more appropriate for areas where large volumes of urine are available within a small area; public toilets, schools and stadia are promising sources of urine.
Magnesium should be available in a suitably soluble form, in sufficient quantities and at an affordable price to allow for an economic operation of the struvite production plant. Whereas direct application of urine may face low social acceptance, struvite, as a urine-derived but odourless product is generally well accepted.
Optimization of low-cost struvite recovery
Complete report on the pilot scale struvite reactor developed and operated in the Kathmandu Valley.
ETTER, B. (2009): Optimization of low-cost struvite recovery. (= Master thesis ). Swiss Federal Institute of Technology Lausanne (EPFL) URL [Accessed: 27.05.2019]Struvite - Recovery from urine at community scale in Nepal
Preliminary research on the pilot scale struvite reactor developed and operated in the Kathmandu Valley.
ETTER, B. (2009): Struvite - Recovery from urine at community scale in Nepal. Duebendorf: Swiss Federal Institute of Aquatic Science and Technology (Eawag). [Acccessed: 29.07.2010] PDFDevelopment of struvite reactors for phosphate recovery from urine in the Kathmandu Valley
How to produce fertilizer from urine: Struvite. Brochure in English/Nepali
Brochure explaining the construction and functioning of the struvite reactor.
EAWAG (2009): How to produce fertilizer from urine: Struvite. Brochure in English/Nepali. Duebendorf: Swiss Federal Institute of Aquatic Science and Technology (EAWAG) URL [Accessed: 27.05.2019]World Toilet Day: Creating Value from Urine. Press Release
Press release on the motivation for struvite recovery in a developing country setting.
EAWAG (2009): World Toilet Day: Creating Value from Urine. Press Release. Duebendorf: Swiss Federal Institute of Aquatic Science and Technology (EAWAG). [Accessed: 29.07.2010] PDFNepal: Pee proudly for healthy vegetables
News feature describing the advantages of recycling nutrients from urine in struvite form.
ETTER, B. (2008): Nepal: Pee proudly for healthy vegetables. IRC Source News. [Accessed: 29.07.2010] PDFStruvite - Recovery from urine at community scale in Nepal
Preliminary research on the pilot scale struvite reactor developed and operated in the Kathmandu Valley.
ETTER, B. (2009): Struvite - Recovery from urine at community scale in Nepal. Duebendorf: Swiss Federal Institute of Aquatic Science and Technology (Eawag). [Acccessed: 29.07.2010] PDFStruvite Recovery from Urine at Community Scale in Nepal Final Project Report Phase I.
Low-cost Struvite Reactor - Construction Manual
The aim of this publication is to present and discuss the design drawings, from which a struvite precipitation reactor can be built. In addition, a breakdown of the building costs and some suggestions for improvements are presented.
ZANDEE, M. ETTER, B. (2011): Low-cost Struvite Reactor - Construction Manual. Duebendorf: Swiss Federal Institute of Aquatic Science and Technology (EAWAG) URL [Accessed: 05.06.2019]Low-cost Struvite Reactor - Operation Manual
The described set-up in this manual addresses a low-cost solution for struvite production for processing the urine produced by up to 1600 citizen.
MEYER, R. ETTER, B. UDERT, K. (2011): Low-cost Struvite Reactor - Operation Manual. Duebendorf: Swiss Federal Institute of Aquatic Science and Technology (EAWAG). [Accessed: 30.11.2011] PDFThe Characterization of Feces and Urine: A Review of the Literature to Inform Advanced Treatment Technology
Development of on-site sanitation facilities that treat excreta require knowledge of the waste stream entering the system. This paper contains data regarding the generation rate and the chemical and physical composition of fresh feces and urine. In addition, the impact on biological and thermal processes, physical separators, and chemical reactions is also assessed.
ROSE, C. ; PARKER, A. ; JEFFERSON, B. ; CARTMELL, E. (2015): The Characterization of Feces and Urine: A Review of the Literature to Inform Advanced Treatment Technology. In: Critical Reviews in Environmental Science and Technology: Volume 45 , 1827-1879. URL [Accessed: 25.11.2015]Optimization of low-cost struvite recovery
Complete report on the pilot scale struvite reactor developed and operated in the Kathmandu Valley.
ETTER, B. (2009): Optimization of low-cost struvite recovery. (= Master thesis ). Swiss Federal Institute of Technology Lausanne (EPFL) URL [Accessed: 27.05.2019]Struvite recovery in Kathmandu: A business model for increased food security
Poster illustrating the potential of operating struvite recovery as a business for independent fertiliser production.
EAWAG (2009): Struvite recovery in Kathmandu: A business model for increased food security. Swiss Federal Institute of Aquatic Science and Technology (EAWAG). [Accessed: 29.07.2010] PDFStruvite recovery from urine in Nepal. Poster (in Nepali/English)
- Poster with an overview on the struvite production process in Nepal, process inputs and outputs.
STruvite from Urine in Nepal (STUN)
Project website on "Struvite recovery from urine in Nepal" with plenty of downloadable resources.
Ostara Nutrient Recovery Technologies Inc.
Commercial large-scale production of struvite from sludge digester supernatant in North America.
Phosphorus Futures
Website of the Global Phosphorus Research Initiative (GPRI), a collaboration between independent research institutes in Europe, Australia and North America. The main objective of the GPRI is to facilitate quality interdisciplinary research on global phosphorus security for future food production. In addition to research, the GPRI also facilitates networking, dialogue and awareness raising among policy makers, industry, scientists and the community on the implications of global phosphorus scarcity and possible solutions.