Executive Summary
As an effective yet inexpensive disinfectant, chlorination is the most commonly used disinfection method in drinking water treatment plants all over the world. Chlorine kills pathogens and oxidises iron, manganese, and taste and odour compounds in water. It can be added to water as a primary disinfection as pre-chlorination, between sedimentation and filtration, or as a final treatment step before distribution. It can also be added as a secondary disinfection to water leaving the plant or within distribution networks to prevent recontamination and ensure drinking water safety. While it is effective in improving water quality and killing pathogens, chlorination may lead to the formation of by-products that can be toxic or cause taste and odour problems for drinking water, so care should be taken to prevent the formation of these compounds.
Introduction
The control of infectious diseases through clean water and improved sanitation is one of the most important public health achievements of the 20th century. The use of chlorine in the treatment of drinking water played a major role in reducing or even virtually eliminating waterborne diseases in developed countries, such as typhoid fever, cholera, dysentery, and other gastroenteritic diseases.
Centralised chlorination involves the use of chlorine for disinfection within a centralised drinking water supply system, either as primary or secondary disinfection. Chlorine is the most commonly used disinfectant in the world for treating drinking water worldwide, due to its advantages of being an inexpensive yet effective water purification method.
How does it work?
Chlorine can be added to water in different forms, depending on the pH conditions required and the available storage options. The three most common types of chlorine used in water treatment are: chlorine gas, sodium hypochlorite, and calcium hypochlorite.
Once added to the water, chlorine kills microorganisms such as bacteria, algae, and fungi (read more about pathogens and contaminants). It kills cells by first damaging the cell membrane, entering the cell, and disrupting cell respiration and DNA activity, two processes that are necessary for cell survival. In addition to killing microorganisms, chlorine also oxidises iron, manganese, taste and odour compounds, removes colour in the water, and destroys hydrogen sulphide.
What is it used for?
The ability of chlorine to kill many types of microorganisms growing in water makes chlorination suitable for the following uses:
- To prevent algal, fungal, and bacterial growth (see pathogens and contaminants).
- To control slime growth in distribution systems (to learn about residual chlorine, see also preventing recontamination).
- To maintain clean filter media at the treatment plant (see also slow and rapid sand filtration).
- To restore and preserve pipeline capacity (to learn about residual chlorine, see also preventing recontamination).
- To restore well capacity, to disinfect water mains (see also well development and rehabilitation).
- To control taste and odours
How can it be used?
At household level
Chlorination is one of many household water treatment systems that can be done to ensure safe drinking water at the point of use. To learn more about how chlorination can be done at a household level, see point of use chlorination.
At municipal level
Chlorination is the most common disinfection method in drinking water treatment plants, and can be done at any stage throughout the water treatment process. Each point of chlorine application will control a different water contaminant concern, thus offering a complete spectrum of treatment from the time the water enters the treatment facility to the time it leaves.
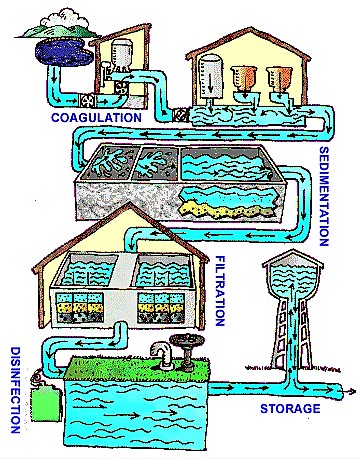
The chlorination process is integrated into water treatment plants as a primary (within a water purification plant) or secondary (within distribution system) disinfection method.
Primary disinfection
Primary disinfection is the application of a disinfectant in the drinking water treatment plant. The amount of chlorine needed and time needed to react and disinfect is called the Contact Time (CT), and is a product of the concentration of residual chlorine (mg/l) and the disinfectant contact time. CT values required to achieve the necessary disinfection depends on the microorganism targeted, pH, and temperature. Other design factors influencing the amount of chlorine required are contact chamber design, adequate mixing, and the presence of sunlight.
The following are possibilities for chlorination as a primary disinfection method:
Pre-chlorination
In pre-chlorination, chlorine is applied to the water almost immediately after it enters the treatment facility to eliminate algae and other organisms from water so they won’t cause a problem in later treatment stages. Pre-chlorination is found to remove tastes and odours and control biological growth throughout the water treatment system, thus preventing growth in the sedimentation tanks and the filtration media. The addition of chlorine also oxidises any iron, manganese and/or hydrogen sulphide that are present, so that they too can be removed in the sedimentation and filtration steps.
After sedimentation and before filtration
This controls the biological growth, removes iron and manganese, removes taste and odours, controls algae growth, and removes the colour from the water.
Final treatment step
The most common stage for chlorination is as a final treatment step to disinfect the water and maintain chlorine residuals that will remain in the water as it travels through the distribution system. Chlorinating as a final step is more economical because a lower CT value is required, as by the time the water has been through sedimentation and filtration, a lot of the unwanted organisms have been removed, so less chlorine and a shorter contact time is required to achieve the same effectiveness.
Secondary disinfection
Secondary disinfection may be applied to the treated water as it leaves the treatment plant or at rechlorination points throughout the distribution system, to introduce and maintain a chlorine residual in the drinking water distribution system. Overall, a chlorine residual provides two main benefits:
- It can limit the growth of biofilm within the distribution system and its associated taste and odour problems.
- A rapid drop in disinfectant residual may provide an immediate indication of treatment process malfunction or a break in the integrity of the distribution system
A chlorine residual may also reduce the risk of recontamination in the event of an intrusion into the distribution system.
Optimisation
Chlorination disinfection by-products (CDBPs)
A number of different by-products can be produced from reactions in the chlorination process. Some by-products, such as chloramines, are beneficial to the disinfection process because they also have disinfecting properties. However, there are several undesired compounds that may be produced from chlorine reacting with natural organic matter such as humic and fulvic acids, which are generated from the decay of organic matter:
- Trihalomethanes (THMs): considered carcinogenic. The trihalomethane of most concern is chloroform (also called trichloromethane). Chronic exposure may cause damage to the liver and kidneys.
- Haloacetic acids (HAAs; includes trichloroacetic acid, dichloroactic acid):Trichloracetic acid is produced commercially for use as a herbicide and is also produced in drinking water, but is not classified as a carcinogen for humans. Dichloroacetic acid is an irritant, corrosive, and destructive against mucous membranes.
- Haloacetonitriles: were used as pesticides in the past, but are no longer manufactured, and form from chlorine, natural organic matter, and bromide.
- Chlorophenols: cause taste and odour problems. They are toxic, and when present in higher concentrations, affect the respiration and energy storage process in the body.
In order to avoid the formation of CDBPs, it is recommended to remove organic precursors or optimise the treatment system to so that chlorine is added after organic precursors have been removed.
Taste and odour problems
While chlorination can help improve taste and odour through the reaction with organic materials and iron, it can also generate chlorinous flavours caused by the presence of the disinfectant itself or by the occurrence of other CDBPs formed by the reaction with other compounds in the water. For example, the reaction of chlorine with certain nitrogen compounds (e.g., amino acids, ammonium, urea) present in source water may lead to the formation of strong-smelling compounds such as aldehydes, nitriles, and some chloramines, which can cause pronounced chlorinous tastes and odours, sometimes even at very low levels. Chlorophenols can also be formed at the plant or in the distribution system and can impart taste and odours to the water.
The consequence of these compounds in drinking water is consumer dissatisfaction, turning to other water sources (such as bottled water), purchasing home filtration devices (MACKEY 2009), and the rejection of a water source that is actually safe to drink. This is particularly a problem when no other safe drinking water sources are available.
While chlorination can result in CDBPs that pose health risks and cause taste and odour problems, risks to health from these by-products are extremely small in comparison with the risks associated with inadequate disinfection.
Health aspects
Chlorine itself is non-toxic, and while drinking water typically contains around 1 mg/L, even 50 mg/L has been shown to have no health impact. However, some CDBPs are carcinogenic and should be avoided by removing organic precursors before chlorination. Despite these risks, the effectiveness in killing pathogens far outweighs the risk of CDBPs.
While most microorgansisms are rapidly deactivated by chlorine (e.g. E. coli), others are chlorine-resistant (e.g. Giardia and Cryptosporidium), and thus are not killed when chlorine is added to water. If these pathogens are present in large concentrations, additional treatment is needed such as membrane filtration or boiling (for more information pathogens in drinking water, see pathogens and contaminants).
Cost
Chlorine is a low-cost way to effectively disinfect drinking water. Centralised chlorination as primary disinfection is most economical when added as a final treatment step, as less chlorine is needed to achieve the same level of disinfection.
Operation and maintenance
Chlorination is an effective water purification method, but the presence of inorganic compounds (e.g. iron, manganese, etc.) or natural organic matter may result in decreased efficiency in pathogen deactivation and in the formation of harmful CDBPs. Therefore, removal of these compounds before the addition of chlorine is recommended, or other water purification methods preferentially employed, such as solar disinfection (see solar pasteurisation, UV tubes, and SODIS), membrane filtration, or boiling to kill pathogens.
To ensure effectiveness and prevent recontamination, a chlorine residual should be maintained throughout the distribution system. Water leaving a treatment plant should be tested daily for chlorine residual, turbidity, total coliforms, and E. coli to confirm the microbiological safety of the supply. In the distribution system, the presence of adequate chlorine residuals should be confirmed when sampling for total coliforms and E. coli.
- Chlorination is the most widely used water disinfectant in water treatment plants around the world due to it being cheap and effective. However, recently it is being more and more replaced by ozonation.
- If there are high concentrations of contaminants in water that react with chlorine (such as natural organic matter, iron, manganese, etc.), additional treatment should take place before chlorination to ensure efficacy and reduce the risk of formation of CDBPs.
Influence of Taste and Odor on Consumer Perception of Tap Water Quality and Safety
What is Chlorination?
Water Treatment Process
Chlorine Disinfection By-Products and Waterborne Disease. The Need for Balance is Essential
This magazine article focuses on the formation and risks associated with various chlorine disinfection by-products, emphasizing the fact that the risks associated with these by-products must be balanced with the benefit of effectively eliminating water borne diseases.
BHARDWAJ, V. (2004): Chlorine Disinfection By-Products and Waterborne Disease. The Need for Balance is Essential. In: NESC (2004): A Tie That Binds. Public Drinking Water and Public Health. Morgantown: . URL [Accessed: 19.10.2012]The Practice of Chlorination. Application, Efficacy, Problems and Alternatives
This position paper provides an overview discussion on the practice of chlorination in water treatment. The discussion covers the following topics: general chlorination practice, chlorine disinfection capabilities, by-product formation, current regulations and use of alternative oxidants for disinfection.
BRAGHETTA, A. JACANGELO, J. TRUSSELL, R.R. MEHEUS, J. (1997): The Practice of Chlorination. Application, Efficacy, Problems and Alternatives. Colombo: The International Water Association (IWA) URL [Accessed: 22.05.2019]Drinking Water Chlorination. A Review of Disinfection Issues and Practices
This document gives an overview on the role of chlorination in disinfecting drinking water. It includes information on chlorine and waterborne disease, disinfection by-products, and compares chlorine to alternative disinfection methods such as ozone and ultraviolet radiation.
C4 (2003): Drinking Water Chlorination. A Review of Disinfection Issues and Practices. Chlorine Chemistry Council (C3) and Canadian Chlorine Coordinating Committee (C4) URL [Accessed: 22.05.2019]Basic Information about Disinfectants in Drinking Water. Chloramine, Chlorine and Chlorine Dioxide
This webpage by the U.S. EPA gives basic information about the role of chlorine in disinfecting drinking water in the U.S., including the types of chlorine added and the formation of chlorine disinfection by-products.
EPA (2012): Basic Information about Disinfectants in Drinking Water. Chloramine, Chlorine and Chlorine Dioxide. Washington, D.C.: U.S.: Environmental Protection Agency (EPA) URL [Accessed: 22.05.2019]Consumer Preferences. An Overview
This document provides information about the consumer perception of drinking water regarding water quality, taste, and odor and how these factors influence consumer perception about the safety and quality of drinking water.
FIFE-SCHAW, C. KELAY, T. VLOERBERGH, I. CHENOWETH, J. MORRISON, G. LUNDEHN, C. (2006): Consumer Preferences. An Overview. Technology Enabled Universal Access to Safe Water (TECHNEAU). [Accessed: 19.10.2012] PDFChlorination. Tech Brief
This technical factsheet gives basic information about chlorine, how chlorine is added to our drinking water, and about chlorine disinfection by-products.
LINDSAY,L. (n.y): Chlorination. Tech Brief. Morgantown: The National Environmental Services Center URL [Accessed: 19.10.2012]Drinking water chlorination
This 8-pages information paper highlights chlorine’s critical role in providing safe drinking water; the potential health and environmental effects of chlorine and disinfection by-products; and considerations for selecting disinfection methods.
WORLD CHLORINE COUNCIL (2008): Drinking water chlorination. Position Paper URL [Accessed: 18.05.2019]Central Municipality. User of Chlorine Dioxide Improves Taste and Odor and Controls THMs
This case study describes how a drinking water treatment plant reduced the number of complaints about the taste and odour of drinking water and reduced the formation of trihalomethanes (a disinfection by-product) by pre-chlorinating raw water with chlorine dioxide.
SIEMENS WATER TECHNOLOGIES (2008): Central Municipality. User of Chlorine Dioxide Improves Taste and Odor and Controls THMs. USA: Siemens. [Accessed: 26.10.2012] PDFRocky Mountain Drinking Water Plant Removes Manganese and Improves Turbidity with Chlorine Dioxide
This short case study describes how a drinking water treatment plant was able to remove manganese and reduce turbidity by applying chlorine dioxide to raw water as a pre-oxidant.
SIEMENS (2011): Rocky Mountain Drinking Water Plant Removes Manganese and Improves Turbidity with Chlorine Dioxide. USA: Siemens URL [Accessed: 22.05.2019]The Dutch Secret. How to provide safe drinking water without chlorine in the Netherlands
This article describes how safe water is supplied and distributed in the Netherlands without the use of chlorine in primary or secondary disinfection steps.
SMEETS, P. ; MEDEMA, G. ; DIJK, J. van (2009): The Dutch Secret. How to provide safe drinking water without chlorine in the Netherlands. In: Drinking Water Engineering and Science : Volume 2 , 1-14. URL [Accessed: 23.10.2012]Disinfection
This training guide provides practical information on the chemistry behind chlorination, application of chlorination in a treatment plant, and chlorine safety, including a section of study questions.
RAGSDALE AND ASSOCIATES (2002): Disinfection. Chapter 5. In: RAGSDALE AND ASSOCIATES (2002): New Mexico Water Systems Operator Certification Study Guide. Santa Fe: . URL [Accessed: 22.05.2019]Virtual Tour of a Drinking Water Plant
Your public water system is the first line of defense against waterborne disease. View step-by-step how water is treated and delivered to your home or business as water that is safe to drink. Your drinking water is inexpensive compared to other household costs. Learn why it is important to keep the environment clean and find out what you can do to protect our nation's sources of drinking water. Fun facts and classroom activities are also featured. This Video can be viewed in English or Spanish.
US EPA (2012): Virtual Tour of a Drinking Water Plant. URL [Accessed: 22.05.2019]The water treatment process
This informational video, produced by Severn Trent Water, goes through the entire process of centralised drinking water treatment from raw water source to clean drinking water. In the drinking water scheme presented, chlorine is added as a final disinfection step as the water leaves the plant to the distribution network.