Resumen ejecutivo
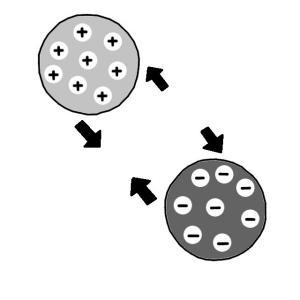
Ion exchange is a water treatment method where one or more undesirable ionic contaminants are removed from water by exchange with another non-objectionable, or less objectionable ionic substance. Both the contaminant and the exchanged substance must be dissolved and have the same type of electrical charge (positive or negative). A typical example of ion exchange is a process called “water softening” aiming to reduce calcium and magnesium content. Nevertheless, ion exchange is also efficient in removing toxic metals from water.
| Entradas | Salidas |
|---|---|
Treated Water |
Introduction
In 1850, Thomas and Way performed some of the first scientific research that indicated the existence of an ion exchange process. In their experiment, a solution of ammonium sulfate was passed through soil. The filtrate collected was composed of calcium sulfate instead of ammonium sulfate (KELLER 2005). The importance of this discovery (in ion exchange terms) was not fully understood until later in that decade, when it was found that this reaction was reversible. Ion exchange was then primary used to soften water.
The presence of calcium and/or magnesium in water results in water being considered “hard”. Calcium and magnesium ions in water react with heat, metallic plumbing and chemical agents such as detergents to decrease the effectiveness of nearly any cleaning task. Hard water can be softened using an ion exchange softening process (SKIPTON 2008).
Ion exchange processes can also remove various charged atoms or molecules (ions) such as nitrates, fluoride, sulphates, perchlorate, iron and manganese ions as well as toxic metals (radium, uranium, chromium, etc.) from water.
The most typical application of ion exchange is the preparation of high purity water for industrial applications, water softening, recovery or removal of metals in the chemical industry.
Ion exchange resins
(Adapted from NEUMANN and FATULA 2009)
Synthetic and industrially produced ion exchange resins consist of small, microporous beads that are insoluble in water and organic solvents. The most widely used base-materials are polystyrene and polyacrylate. The diameter of the beads is in the range of 0.3 to 1.3 mm. The beads are composed of around 50% water, which is dispersed in the gel-structured compartments of the material.
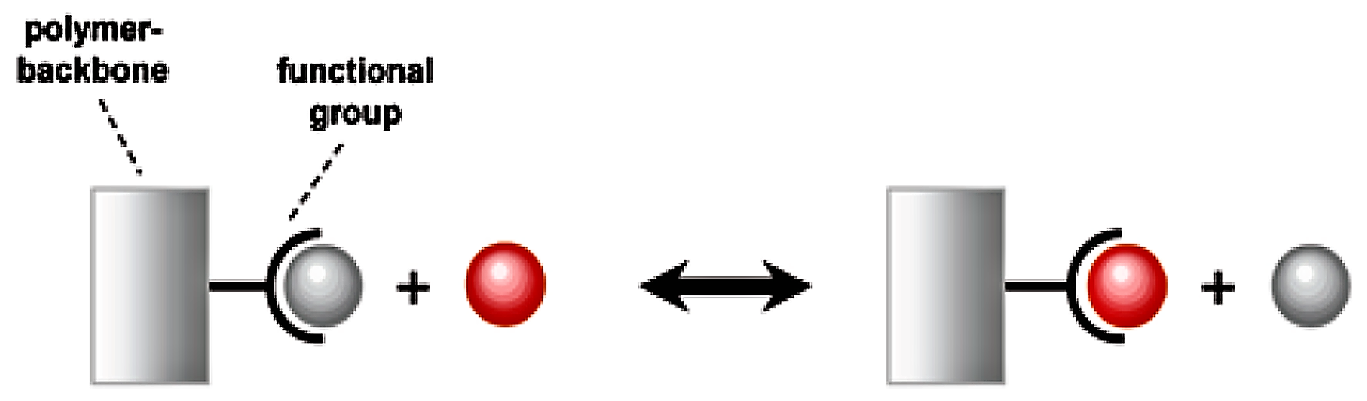
Since water is dispersed homogenously throughout the bead, water-soluble materials can move freely in and out. To each of the monomer units of the polymer, so called “functional groups” are attached. These functional groups can interact with water-soluble species, especially with ions. Ions are either positively charged (cations) or negatively charged (anions). Since the functional groups are also charged, the interaction between ions and functional groups is exhibited via electrostatic forces. Positively charged functional groups interact with anions and negatively charged functional groups interact with cations.
The binding force between the functional group and the attached ion is relatively weak. The exchange can be reversed by another ion passing across the functional group. This process can be repeated continually, with one exchange reaction following another.
Ion exchange process
(Adapted from SKIPTON 2008)
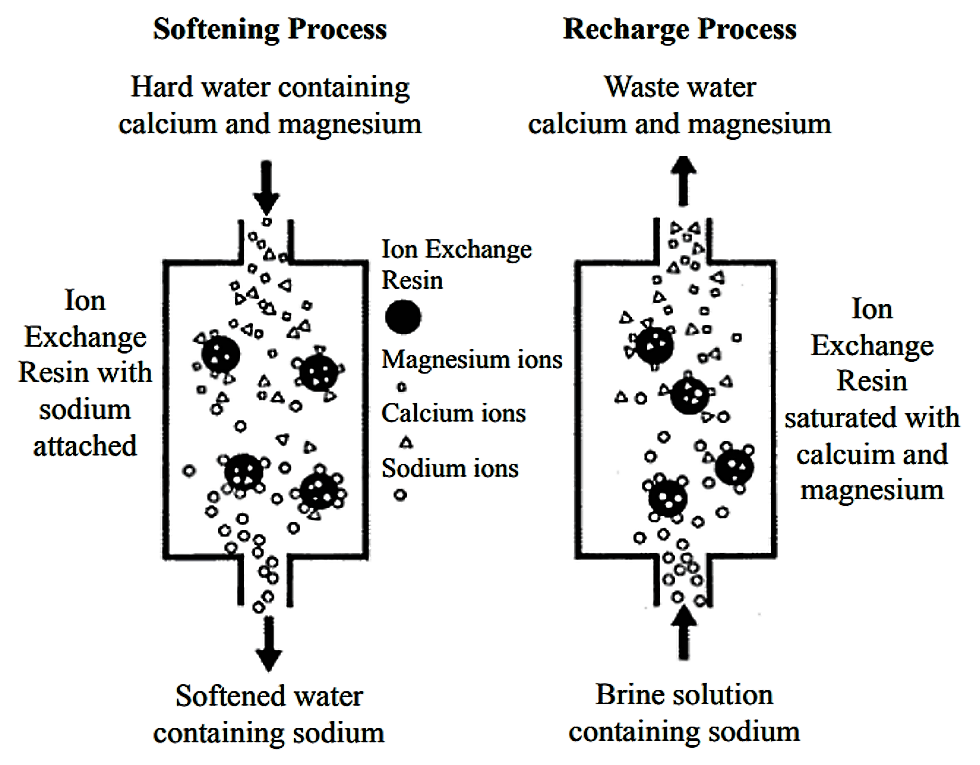
The main component of ion exchange equipment is a microporous exchange resin, which is supersaturated with a loosely held solution. For water softening, this is usually done with sulfonated polystyrene beds that are supersaturated with sodium to cover the bed surface. As water passes through this resin bed, ions attach to the resin beads releasing the loosely held solution into the water.
After a time, the beds become saturated and the exchange resin must be regenerated or recharged. To regenerate, the ion exchange resin is flushed with a salt brine solution. The sodium ions in the salt brine solution are exchanged with the ions, which are flushed out with wastewater.
Operation and maintenance
(Adapted from SKIPTON 2008)
Maintenance of water softening equipment is somewhat dependent on the type of softener. Some degree of monitoring or managing the regeneration process is generally required. Adequate backwashing of the resin bed is important to ensure the regeneration of the unit. However, regeneration creates wastewater.
Costs
The costs for ion exchange systems are very variable depending on scale and region. Moreover, costs depend on pretreatment requirements, discharge requirements and utilisation.
Health aspects
People on restricted sodium diets due to health reasons should account for increased intake through softened water. Drinking and cooking with softened water is often avoided by having a cold water line to the kitchen tap that bypasses the water softener. This provides hard water for drinking cooking and other uses. It is not recommended to repeatedly use softened water for plants, lawns or gardens due to the sodium content.
At a glance
| Working principle | Undesirable ionic contaminants are removed from water by exchange with another non-objectionable, or less objectionable ionic substance. |
| Capacity/adequacy | Relatively simple technology. |
| Performance | Efficient technology to remove ionic substances from water and to soften water. |
| Costs | Relatively low costs. |
| Self-help compatibility | Monitoring is necessary to manage the regeneration process. |
| O&M | Ion exchange resin must be regenerated regularly. |
| Reliability | Reliable if ion exchange resin regenerated properly. |
| Main strength | Efficient to remove dissolved inorganics. |
| Main weakness | Do not remove particles or bacteria. |
The most common applications of ion exchangers are water softening (remove calcium and magnesium ions), water demineralisation (removal of all ions), and de-alkalisation (removal of bicarbonates). Cation exchange resins can also remove most positively charged ions in water such as iron, lead, radium, barium, aluminium and copper among others. Anionic exchange units can remove nitrate, sulfate, and other negatively charged atoms (called anions). Researchers are developing resins to selectively remove nitrate more efficiently than can currently be done.Ion exchangers are also used to remove or recover metal ions from wastewater in the chemical industry. Some contaminants (such as arsenic, fluoride, lithium ions) are difficult to remove with ion exchange due to a poor selectivity of the resins.
Ion exchangers are also used to remove or recover metal ions from wastewater in the chemical industry. Some contaminants (such as arsenic, fluoride, lithium ions) are difficult to remove with ion exchange due to a poor selectivity of the resins.
Basic Ion Exchange for Residential Water Treatment Part 1
This series of articles proposes a general overview of many aspects related to ion exchange for residential water treatment. Among other, historic aspects, manufacturing process, softening process, technical aspects, applications in toxic metallic ions removal are covered.
KELLER, M.C. (2005): Basic Ion Exchange for Residential Water Treatment Part 1. Entradas: Water Conditioning and Purification: URL [Visita: 24.05.2019]Principles of Ion Exchange in Wastewater Treatment
Drinking Water Treatment: Water Softening (Ion Exchange)
Integrated Ion Exchange Regeneration Process for Perchlorate in Drinking Water
This report describes an evaluation of an integrated ion exchange regeneration process for perchlorate treatment in drinking water. Integrated ion exchange combines: conventional ion exchange with perchlorate selective resin for wellhead treatment of perchlorate contaminated water, regeneration of resin using tetrachloroferrate (FeCl4-) anion and then returning the resin to service, and the destruction or disposal of perchlorate recovered from the resin.
DOD ; SERDP ; ESTCP (2010): Integrated Ion Exchange Regeneration Process for Perchlorate in Drinking Water. Alexandria (USA): US Department of Defense (DoD), Strategic Environmental Research and Development Program (SERDP), Environmental Security Technology Certification Program (ESTCP) URL [Visita: 24.05.2019]Basic Ion Exchange for Residential Water Treatment Part 1
This series of articles proposes a general overview of many aspects related to ion exchange for residential water treatment. Among other, historic aspects, manufacturing process, softening process, technical aspects, applications in toxic metallic ions removal are covered.
KELLER, M.C. (2005): Basic Ion Exchange for Residential Water Treatment Part 1. Entradas: Water Conditioning and Purification: URL [Visita: 24.05.2019]Basic Ion Exchange for Residential Water Treatment Part 2
This series of articles proposes a general overview of many aspects related to ion exchange for residential water treatment. Among other, historic aspects, manufacturing process, softening process, technical aspects, applications in toxic metallic ions removal are covered.
KELLER, M.C. (2005): Basic Ion Exchange for Residential Water Treatment Part 2. Entradas: Water Conditioning and Purification: URL [Visita: 24.05.2019]Basic Ion Exchange for Residential Water Treatment Part 3
This series of articles proposes a general overview of many aspects related to ion exchange for residential water treatment. Among other, historic aspects, manufacturing process, softening process, technical aspects, applications in toxic metallic ions removal are covered.
KELLER, M.C. (2005): Basic Ion Exchange for Residential Water Treatment Part 3. Entradas: Water Conditioning and Purification. [Accessed: 27.02.2012]: PDFIon Exchange Process
This summary of ion exchange technologies has a special emphasis on ion exchange materials and processes.
NALCO (1998): Ion Exchange Process. Entradas: Online Water Treatment: URL [Visita: 24.02.2019]Magnetic Ion Exchange: Is there a Potential for International Development
Magnetic ion exchange (MIEX) is an ion exchange resin developed as an additive to existing water treatment plants where additional organic matter is to be removed. The smaller size, magnetic properties and simple regeneration using NaCl distinguish MIEX from conventional ion exchange resins. Its use in international development applications is investigated in this review article.
NEALE, P.A. ; SCHAFER, A.I. (2010): Magnetic Ion Exchange: Is there a Potential for International Development. Entradas: Desalination : Volume 251 , 160-168. URL [Visita: 24.05.2019]Ion Exchange Treatment of Drinking Water
This fact sheet summarises aspects related to ion exchange to soften water with an emphasis on reduction of salt usage.
NHDES (2009): Ion Exchange Treatment of Drinking Water . New Hampshire: New Hampshire Department of Environmental Services (NHDES) URL [Visita: 27.02.2012]Ion Exchange Resin for Removing Hexavalent Chromium from Ground Water at Treatment Facility C: Data on Removal Capacity, Regeneration Efficiency, and Operation
This case study is about the removal of chromium from groundwater by means of ion exchange resin.
BAHOWICK, S. ; DOBIE, D. ; KUMAMOTO, G. (1993): Ion Exchange Resin for Removing Hexavalent Chromium from Ground Water at Treatment Facility C: Data on Removal Capacity, Regeneration Efficiency, and Operation. Entradas: Environmental Restoration Division, Lawrence Livermore National Laboratory. [Accessed: 27.02.2012]: PDFWorld First Magnetic Ion Exchange (MIEX) Water Treatment Plant to be Installed in Western Australia
This article describes for the first time the use of magnetic ion exchange for improving Wanneroo (Australia) Groundwater Treatment Plant treatment process.
CADEE, K. ; O’LEARY, B. ; SMITH, P. ; SLUNJSKI, M. ; BOURKE, M. (2000): World First Magnetic Ion Exchange (MIEX) Water Treatment Plant to be Installed in Western Australia. Entradas: miexresin.com: URL [Visita: 24.05.2019]Ion Exchange
This presentation describes different aspects of ion exchange technology such as physical process, application, and theory.
ARMENANTE, P.M. (n.y): Ion Exchange. Entradas: New Jersey Institute of Technology. [Accessed: 27.02.2012]: PDFGuidelines for Selecting Resin Ion Exchange or Reverse Osmosis for Feed Water Demineralisation
Both reverse osmosis and ion exchange technologies are well established and have reached advanced levels of development. Comparison of the two processes for water demineralisation can therefore be reliably made and cost comparisons can be carried out on a case-by-case basis. This document examines the significant factors that should be taken into account in the comparison and gives guidelines for decision making.
PUROLITE INTERNATIONAL (2003): Guidelines for Selecting Resin Ion Exchange or Reverse Osmosis for Feed Water Demineralisation. Entradas: Purolite.com: URL [Visita: 24.05.2019]Ion exchangers and their use in waste water treatment
This web link describes the use of ion exchangers and their use in wastewater treatment, including theoretical and practical information.
Basic ion exchange processes in water treatment
This website is about basic ion exchange processes in water treatment with an emphasis on applications such as softening, demineralisation, and nitrates removal.
How To Remove Radioactive Iodine-131 From Drinking Water
This is an article on how to remove radioactive iodine-131 from drinking water, talking about different technologies including ion exchange.